Home>Science>Unveiling The Mind-Blowing Molecular Geometry Of CH3NH2!

Science
Unveiling The Mind-Blowing Molecular Geometry Of CH3NH2!
Published: January 12, 2024
Explore the fascinating molecular geometry of CH3NH2 in this scientific exploration. Uncover the secrets of science with this mind-blowing revelation. Discover more!
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Regretless.com, at no extra cost. Learn more)
Table of Contents
Introduction
In the fascinating world of chemistry, the study of molecular geometry holds a crucial place. Understanding the arrangement of atoms within a molecule provides profound insights into its behavior and properties. One such captivating molecule that has piqued the curiosity of scientists and enthusiasts alike is CH3NH2, also known as methylamine.
Methylamine, with its intriguing molecular structure, has garnered attention for its diverse applications and significant role in various chemical processes. This compound, composed of carbon, hydrogen, and nitrogen atoms, exhibits a compelling arrangement that contributes to its distinctive characteristics and behavior.
In this comprehensive exploration, we will delve into the mind-blowing molecular geometry of CH3NH2, unraveling its structural intricacies, properties, and real-world applications. By unraveling the enigmatic nature of this compound, we aim to shed light on its significance in the realm of chemistry and its impact on diverse fields, from pharmaceuticals to industrial processes.
Join us on this captivating journey as we unravel the fascinating world of molecular geometry and discover the remarkable attributes of CH3NH2, a compound that continues to intrigue and inspire scientific inquiry and innovation.
Understanding Molecular Geometry
Molecular geometry, a fundamental concept in chemistry, pertains to the three-dimensional arrangement of atoms within a molecule and the spatial relationships between them. It encompasses the bond lengths, bond angles, and overall shape of a molecule, all of which profoundly influence its properties and behavior. By comprehending the molecular geometry of a compound, scientists can gain valuable insights into its reactivity, polarity, and interactions with other molecules.
The arrangement of atoms within a molecule is determined by the bonding pairs and lone pairs of electrons around the central atom, following the principles of valence shell electron pair repulsion (VSEPR) theory. This theory posits that electron pairs in the valence shell of an atom repel each other, leading to the adoption of specific geometric arrangements that minimize repulsion and maximize stability.
The VSEPR theory predicts molecular geometries based on the number of bonding and nonbonding electron pairs around the central atom. These electron pairs repel each other and orient themselves in space to achieve the maximum possible distance from each other, ultimately defining the molecular shape. The resulting molecular geometries, such as linear, trigonal planar, tetrahedral, and more, play a pivotal role in determining a molecule's physical and chemical properties.
Understanding molecular geometry is crucial in elucidating the behavior of molecules in various contexts, including chemical reactions, biological processes, and material science. It provides a framework for predicting molecular properties, such as polarity, solubility, and intermolecular forces, which are vital in understanding the behavior of substances in different environments.
In the case of CH3NH2, the molecular geometry arises from the arrangement of the carbon, hydrogen, and nitrogen atoms, as well as the lone pairs of electrons around the nitrogen atom. By unraveling the intricate spatial arrangement of atoms within this compound, we can gain a deeper understanding of its properties and reactivity, laying the groundwork for its diverse applications in both industrial and scientific settings.
The elucidation of molecular geometry not only enriches our understanding of chemical compounds but also serves as a cornerstone for innovation in fields such as pharmaceuticals, materials science, and environmental engineering. By delving into the captivating world of molecular geometry, we embark on a journey of discovery that unveils the hidden intricacies of the building blocks of matter, paving the way for groundbreaking advancements and transformative insights into the world of chemistry.
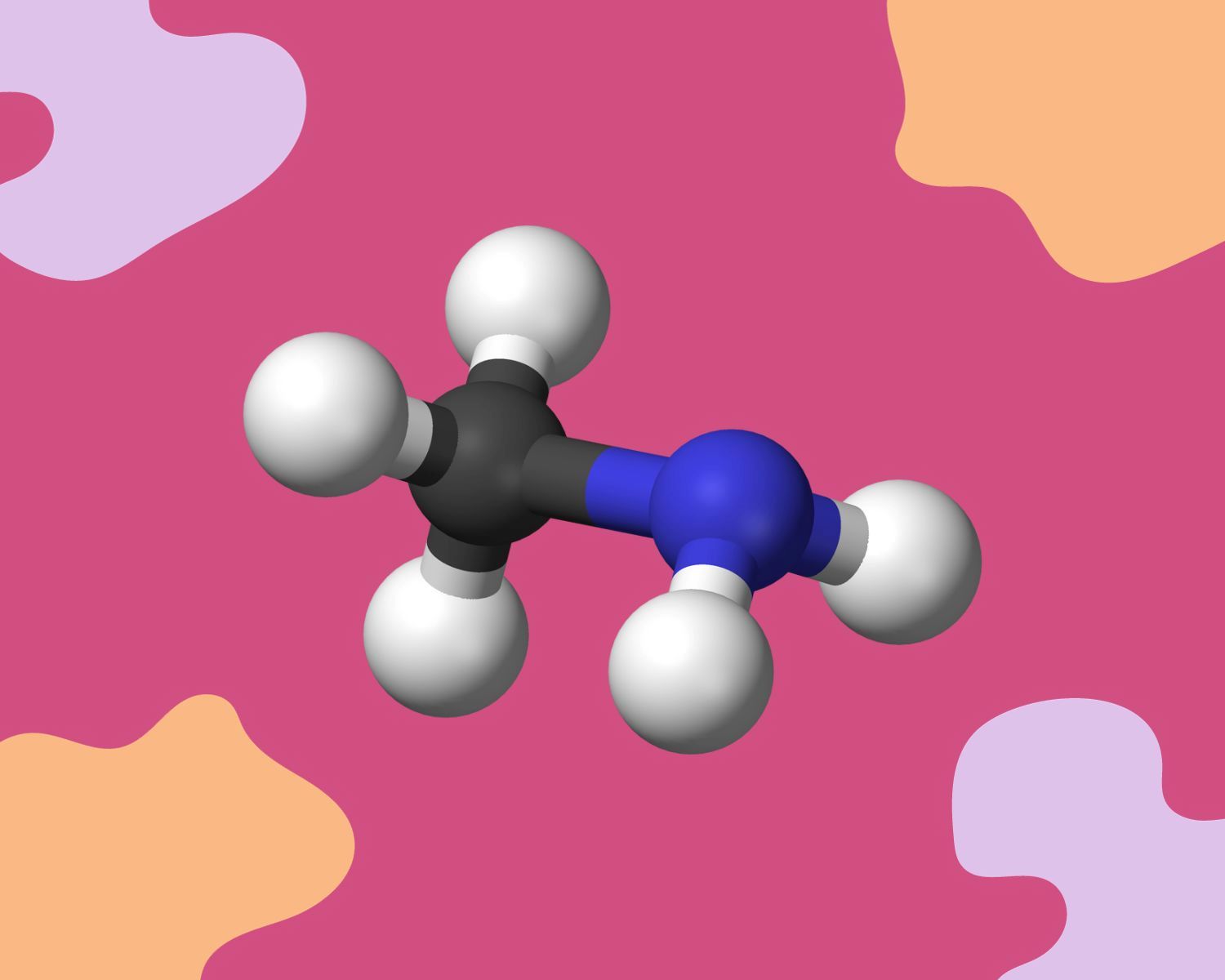
The Structure of CH3NH2
The structure of CH3NH2, also known as methylamine, is a captivating subject that unveils the arrangement of atoms within this intriguing compound. Composed of carbon, hydrogen, and nitrogen atoms, CH3NH2 exhibits a distinctive molecular structure that underpins its properties and behavior. At the heart of its structure lies a central nitrogen atom bonded to three hydrogen atoms and a lone pair of electrons, resulting in a tetrahedral molecular geometry.
The central nitrogen atom in CH3NH2 forms three sigma (σ) bonds with the surrounding hydrogen atoms, leading to a trigonal pyramidal molecular shape. This arrangement is a result of the tetrahedral electron pair geometry around the nitrogen atom, with the lone pair of electrons occupying a distinct position relative to the hydrogen atoms. The presence of the lone pair influences the overall shape of the molecule, contributing to its asymmetry and unique properties.
The structural characteristics of CH3NH2 play a pivotal role in defining its physical and chemical properties. The trigonal pyramidal shape, stemming from the arrangement of atoms and lone pairs, contributes to the molecule's polarity and reactivity. The electronegativity difference between nitrogen and hydrogen atoms leads to a polar covalent bond, with the nitrogen end of the molecule exhibiting a partial negative charge and the hydrogen end displaying a partial positive charge. This polarity influences the molecule's interactions with other substances, impacting its solubility and behavior in various chemical reactions.
Furthermore, the structural arrangement of CH3NH2 holds significance in the context of its role as a building block in organic synthesis and industrial processes. The distinct geometry of the molecule influences its interactions with other compounds, making it a valuable precursor in the production of pharmaceuticals, agrochemicals, and specialty chemicals. The structural intricacies of CH3NH2, coupled with its reactivity and solubility, render it a versatile compound with applications across diverse domains.
In essence, the structure of CH3NH2 encapsulates the intricate interplay of atoms and electron pairs, culminating in a unique molecular geometry that defines its properties and applications. By unraveling the structural nuances of this compound, scientists and researchers gain valuable insights into its behavior and potential uses, paving the way for innovative advancements and discoveries in the realms of chemistry, materials science, and beyond.
Properties of CH3NH2
The properties of CH3NH2, also known as methylamine, stem from its unique molecular structure and composition, giving rise to a diverse array of characteristics that contribute to its significance in various chemical contexts. As a primary amine, CH3NH2 exhibits compelling properties that make it a valuable component in both industrial processes and scientific research.
-
Polarity: CH3NH2 possesses notable polarity due to the electronegativity difference between nitrogen and hydrogen atoms. This results in a polar covalent bond, with the nitrogen end of the molecule bearing a partial negative charge, while the hydrogen end carries a partial positive charge. This polarity influences the interactions of CH3NH2 with other substances, impacting its solubility in polar solvents and its behavior in chemical reactions.
-
Solubility: The polar nature of CH3NH2 contributes to its solubility in water and other polar solvents. This property is significant in various applications, including organic synthesis and chemical reactions where the solubility of the compound plays a crucial role in its reactivity and effectiveness as a reactant.
-
Boiling Point: Methylamine exhibits a relatively low boiling point of -6.3 degrees Celsius, making it a volatile compound. This property is consequential in industrial processes where precise control of temperature and vapor pressure is essential.
-
Odor: CH3NH2 is characterized by a distinct and pungent odor, which is reminiscent of ammonia. This property is noteworthy in practical applications, such as in the production of certain chemicals and the detection of the compound in various settings.
-
Reactivity: As a primary amine, CH3NH2 displays reactivity typical of such functional groups. It undergoes reactions characteristic of primary amines, including nucleophilic substitution, reductive amination, and condensation reactions. This reactivity renders it a versatile building block in organic synthesis and the production of pharmaceuticals and agrochemicals.
-
Toxicity: Methylamine exhibits toxicity at high concentrations, necessitating careful handling and storage in industrial settings. Understanding its toxicological properties is crucial in ensuring safe handling and use in various applications.
-
Industrial Applications: The unique properties of CH3NH2 make it a valuable component in the production of various chemicals, including pharmaceuticals, pesticides, and specialty chemicals. Its solubility, reactivity, and polar nature contribute to its utility as a precursor in organic synthesis and industrial processes.
In essence, the properties of CH3NH2 encompass a wide spectrum of characteristics that are pivotal in understanding its behavior and applications. From its polarity and solubility to its reactivity and industrial relevance, each property contributes to the multifaceted nature of this compound, underscoring its significance in the realms of chemistry, materials science, and industrial chemistry.
Applications of CH3NH2
The versatile nature of CH3NH2, or methylamine, renders it indispensable in a myriad of applications across diverse industries and scientific domains. Its unique properties, including polarity, reactivity, and solubility, contribute to its significance as a valuable building block in organic synthesis, pharmaceuticals, and industrial processes.
Organic Synthesis:
CH3NH2 serves as a fundamental building block in organic synthesis, playing a pivotal role in the production of various compounds, including pharmaceuticals, agrochemicals, and specialty chemicals. Its reactivity as a primary amine enables it to participate in a wide range of chemical reactions, such as nucleophilic substitution, reductive amination, and condensation reactions. This versatility makes CH3NH2 an essential component in the creation of complex organic molecules, contributing to advancements in drug development, materials science, and chemical manufacturing.
Pharmaceutical Industry:
In the pharmaceutical industry, CH3NH2 is utilized in the synthesis of numerous pharmaceutical compounds, including certain medications and active pharmaceutical ingredients (APIs). Its ability to serve as a precursor in the production of pharmaceutical intermediates underscores its critical role in drug synthesis. Additionally, the polar nature and solubility of CH3NH2 make it a valuable component in the formulation of pharmaceutical products, further emphasizing its significance in pharmaceutical research and development.
Agrochemicals:
The production of agrochemicals, such as pesticides and herbicides, often involves the use of CH3NH2 as a key intermediate. Its reactivity and role in organic synthesis enable the creation of specialized agrochemical compounds that contribute to crop protection and agricultural productivity. By serving as a building block for essential agrochemicals, CH3NH2 supports advancements in agricultural science and sustainable farming practices.
Industrial Processes:
CH3NH2 finds extensive use in various industrial processes, including the manufacturing of specialty chemicals, polymers, and chemical intermediates. Its solubility in polar solvents and reactivity in synthetic pathways make it an indispensable component in industrial chemistry. The compound's unique properties and versatility contribute to its application in the creation of diverse industrial products, highlighting its role in driving innovation and progress in industrial sectors.
Research and Development:
The significance of CH3NH2 extends to research and development activities across scientific disciplines. Its utility as a reagent and building block in laboratory settings facilitates the exploration of new chemical compounds and the development of innovative materials. Furthermore, its role in chemical research and experimentation underscores its value in advancing scientific knowledge and fostering discoveries in chemistry and related fields.
In essence, the applications of CH3NH2 span a wide spectrum of industries and scientific endeavors, reflecting its indispensable role as a versatile compound with diverse uses. From organic synthesis and pharmaceuticals to industrial processes and research, CH3NH2 continues to contribute to advancements in chemistry, materials science, and the development of essential products that impact various facets of modern life.
Conclusion
In conclusion, the captivating molecular geometry of CH3NH2, or methylamine, unveils a world of intricate structural arrangements and diverse properties that underpin its significance in the realms of chemistry, industry, and scientific research. The tetrahedral molecular geometry, stemming from the arrangement of carbon, hydrogen, and nitrogen atoms, as well as the lone pair of electrons, defines the unique spatial orientation and characteristics of this compound. This structural intricacy influences its polarity, solubility, reactivity, and applications, making CH3NH2 a versatile and indispensable component in various domains.
The properties of CH3NH2, ranging from its notable polarity and solubility to its reactivity and industrial relevance, collectively contribute to its multifaceted nature and utility in organic synthesis, pharmaceuticals, agrochemicals, and industrial processes. Its distinct odor, toxicity at high concentrations, and low boiling point further underscore the diverse array of characteristics that define its behavior and applications. The compound's role as a building block in organic synthesis, pharmaceutical research, and industrial manufacturing highlights its pivotal position as a fundamental component in the creation of essential products that impact numerous facets of modern life.
Furthermore, the applications of CH3NH2 encompass a wide spectrum of industries and scientific endeavors, reflecting its indispensable role as a versatile compound with diverse uses. From serving as a precursor in drug synthesis and the production of agrochemicals to its application in industrial processes and research, CH3NH2 continues to contribute to advancements in chemistry, materials science, and the development of essential products that impact various facets of modern life.
In essence, the exploration of CH3NH2 and its mind-blowing molecular geometry provides a compelling glimpse into the intricate world of chemical compounds and their profound impact on diverse fields. By unraveling the structural nuances, properties, and applications of CH3NH2, we gain valuable insights into the intricate interplay of atoms and electron pairs, paving the way for innovative advancements and discoveries in the realms of chemistry, materials science, and beyond. As we continue to delve into the captivating world of molecular geometry and chemical compounds, the enigmatic nature of CH3NH2 serves as a testament to the endless possibilities and transformative potential that lie within the realm of chemistry.













