Home>Science>Master The Art Of Counting Atoms In Chemical Formulas!

Science
Master The Art Of Counting Atoms In Chemical Formulas!
Published: January 27, 2024
Learn how to count atoms in chemical formulas and master the art of chemistry with our comprehensive science resources and tutorials. Unlock your potential today!
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Regretless.com, at no extra cost. Learn more)
Table of Contents
Introduction
Welcome to the fascinating world of chemistry, where atoms and molecules form the building blocks of everything around us. Understanding chemical formulas and the art of counting atoms is a fundamental skill for any aspiring chemist. Whether you're a student delving into the wonders of chemistry or a curious mind eager to unravel the mysteries of the elements, mastering the art of counting atoms in chemical formulas is a crucial step in your scientific journey.
In the realm of chemistry, chemical formulas serve as the language through which the composition of substances is communicated. These formulas provide a concise and standardized way to represent the elements and their respective quantities within a compound. By deciphering and counting the atoms present in these formulas, chemists gain valuable insights into the nature and behavior of the substances they represent.
As we embark on this exploration, we will unravel the intricacies of chemical formulas and delve into the essential techniques for counting atoms. Through clear explanations, illustrative examples, and practical exercises, you will gain the confidence and proficiency to navigate the world of chemical formulas with ease.
Whether you're deciphering the formula for water (H2O) or unraveling the complexities of organic compounds, the ability to count atoms accurately is a skill that empowers you to comprehend the composition and properties of diverse substances. So, join us on this enlightening journey as we demystify the art of counting atoms in chemical formulas, equipping you with the knowledge and skills to conquer the captivating realm of chemistry.
Understanding Chemical Formulas
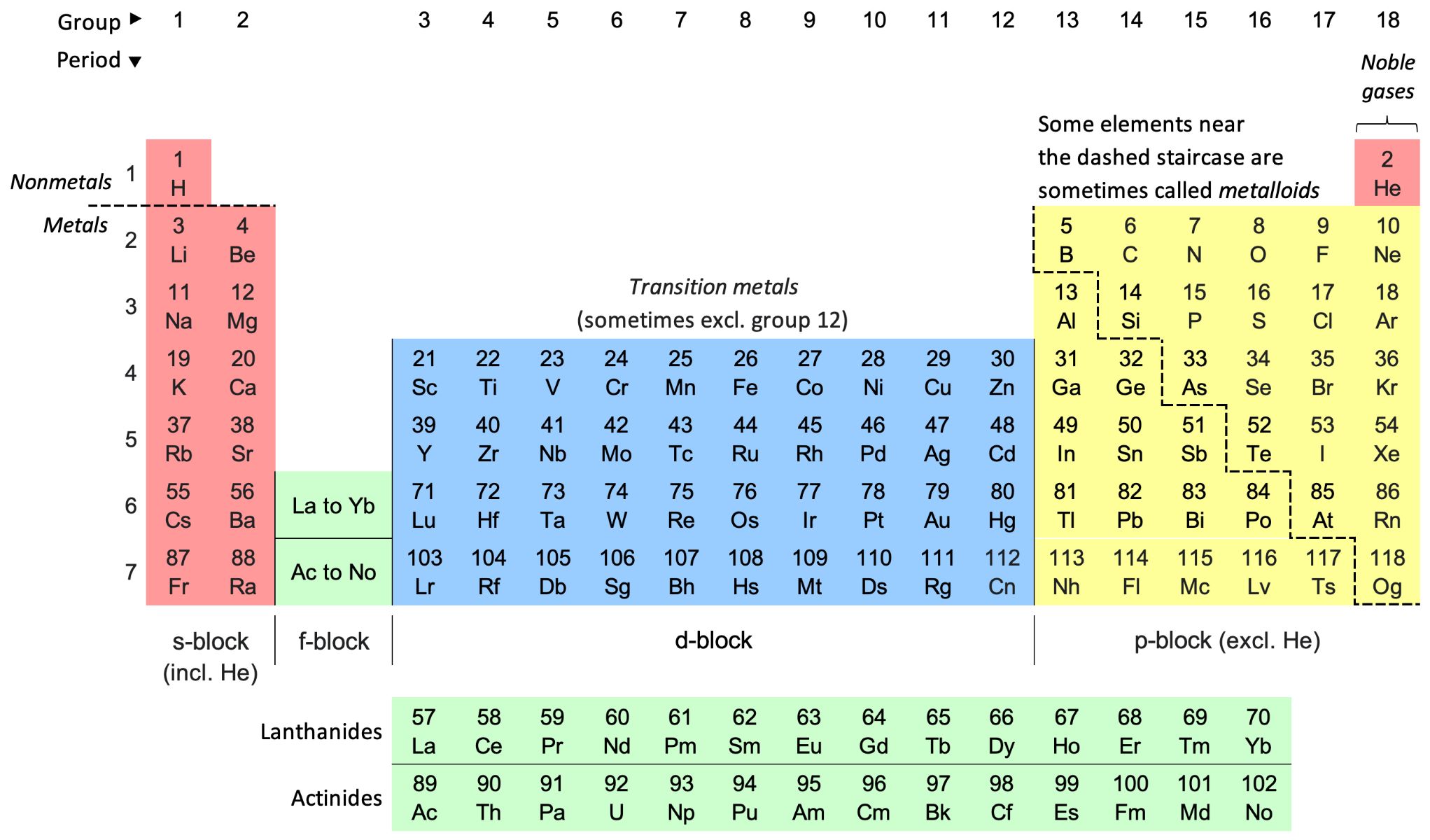
Chemical formulas are the symbolic representations of compounds, conveying vital information about the elements present and their respective quantities within a substance. These concise expressions serve as a universal language in the realm of chemistry, enabling scientists to communicate and comprehend the composition of various compounds.
In a chemical formula, the elements are denoted by their unique symbols, often derived from their Latin names. For instance, 'H' represents hydrogen, 'O' symbolizes oxygen, and 'Na' stands for sodium. The subscripts accompanying these symbols indicate the number of atoms for each element in the compound. For instance, in the formula H2O, the subscript '2' indicates that there are two hydrogen atoms for every one oxygen atom.
Moreover, chemical formulas also provide insights into the bonding and structure of compounds. For instance, the formula H2O not only reveals the elemental composition of water but also signifies the covalent bonds between hydrogen and oxygen atoms. Additionally, parentheses and brackets are used to indicate the presence of polyatomic ions or molecular groups within a compound, further enriching the informational content of chemical formulas.
It's important to note that chemical formulas are not limited to simple compounds; they also extend to complex organic molecules and polymers. These formulas play a pivotal role in elucidating the properties and behaviors of substances, guiding researchers in their quest to understand the intricacies of matter.
By comprehending the significance of chemical formulas and the information they encapsulate, aspiring chemists can unravel the mysteries of compounds and gain a deeper appreciation for the elements that form the very fabric of our world. As we delve deeper into the art of counting atoms in chemical formulas, a solid understanding of these symbolic representations will serve as the foundation for our journey into the captivating realm of chemistry.
The Basics of Counting Atoms
At the heart of chemistry lies the fundamental skill of counting atoms within chemical formulas. This essential process enables scientists to unravel the precise composition of compounds and gain profound insights into their properties and behaviors. To master the art of counting atoms, one must grasp the underlying principles and techniques that govern this intricate task.
When approaching a chemical formula, the first step is to identify the individual elements present and their corresponding symbols. These symbols, derived from the Latin names of the elements, serve as the elemental shorthand in chemical formulas. For instance, 'H' represents hydrogen, 'C' denotes carbon, and 'O' symbolizes oxygen.
Once the elements are identified, the next crucial aspect is discerning the subscripts associated with each element. These subscripts indicate the number of atoms for each element within the compound. It's important to note that if no subscript is explicitly written, it is understood to be '1'. For example, in the formula H2O, there are two hydrogen atoms and one oxygen atom. The subscript '2' applies only to the hydrogen, indicating the presence of two hydrogen atoms in the compound.
In cases where parentheses or brackets are utilized in the chemical formula, the enclosed subscripts apply to all the elements within that group. This grouping mechanism simplifies the process of counting atoms within complex compounds, providing a clear and concise representation of the elemental composition.
Moreover, it's crucial to understand the concept of polyatomic ions, which are tightly bound groups of atoms that carry a net electrical charge. When encountered within a chemical formula, these ions are treated as single entities, and the subscripts outside the parentheses or brackets apply to the entire ion. This nuanced understanding is pivotal in accurately counting atoms within compounds containing polyatomic ions.
By mastering the basics of counting atoms in chemical formulas, aspiring chemists lay a solid foundation for comprehending the intricate structures and behaviors of diverse compounds. This foundational skill not only enables them to decode the elemental composition of substances but also equips them with the ability to predict and understand the myriad interactions and transformations that govern the realm of chemistry.
Practice Problems
Now, let's put our newfound knowledge of counting atoms in chemical formulas to the test through a series of engaging practice problems. These exercises will not only reinforce your understanding but also hone your skills in deciphering and counting atoms within diverse compounds. So, roll up your sleeves, sharpen your pencils, and get ready to tackle these illuminating challenges.
-
Counting Atoms in Simple Compounds
- Given the chemical formula H2O, determine the total number of atoms for each element in the compound.
- Solution: In H2O, there are 2 hydrogen atoms and 1 oxygen atom.
-
Deciphering Complex Formulas
- Unravel the elemental composition of the compound Na2CO3.
- Solution: In Na2CO3, there are 2 sodium atoms, 1 carbon atom, and 3 oxygen atoms.
-
Handling Parentheses and Subscripts
- Delve into the formula (NH4)2SO4 and identify the total count of each element.
- Solution: In (NH4)2SO4, there are 2 nitrogen atoms, 8 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms.
-
Navigating Polyatomic Ions
- Explore the compound Ca(NO3)2 and determine the individual atom count.
- Solution: In Ca(NO3)2, there are 1 calcium atom, 2 nitrogen atoms, and 6 oxygen atoms.
-
Challenging Compound Analysis
- Decipher the formula C6H12O6 and calculate the total number of each atom present.
- Solution: In C6H12O6, there are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
By engaging with these practice problems, you have fortified your ability to decipher and count atoms within chemical formulas. These exercises have equipped you with the proficiency to navigate through simple and complex compounds, unraveling their elemental compositions with precision and clarity. As you continue to immerse yourself in the captivating realm of chemistry, the mastery of counting atoms will serve as a valuable skill, empowering you to unravel the mysteries of matter and delve deeper into the wonders of chemical compounds.
So, embrace these practice problems as stepping stones in your journey to mastering the art of counting atoms, and let the illuminating world of chemistry unfold before you.
Tips for Counting Atoms
Mastering the art of counting atoms in chemical formulas requires attention to detail and a solid understanding of the underlying principles. Here are some valuable tips to enhance your proficiency in counting atoms and deciphering the elemental compositions of diverse compounds:
1. Carefully Identify Elements and Subscripts
When approaching a chemical formula, begin by clearly identifying the elements present and their corresponding symbols. Pay close attention to the subscripts accompanying each element, as these indicate the number of atoms for that particular element within the compound. Ensuring accuracy in identifying elements and their respective quantities is the cornerstone of precise atom counting.
2. Utilize Grouping Mechanisms
In complex chemical formulas, the use of parentheses and brackets serves as a valuable grouping mechanism. When encountering these symbols, recognize that the subscripts within the parentheses or brackets apply to all the elements within that group. Effectively leveraging this grouping mechanism simplifies the process of counting atoms in intricate compounds, facilitating a systematic and accurate approach.
3. Understand the Role of Polyatomic Ions
Polyatomic ions, tightly bound groups of atoms carrying a net electrical charge, are integral components of many chemical compounds. When encountering polyatomic ions within a formula, treat them as single entities and apply the subscripts outside the parentheses or brackets to the entire ion. This nuanced understanding is crucial in accurately counting atoms within compounds containing polyatomic ions.
4. Practice with Diverse Compounds
Engaging in regular practice with a variety of chemical formulas, ranging from simple to complex compounds, is essential for honing your atom counting skills. By tackling diverse examples and practice problems, you develop the confidence and proficiency to decipher and count atoms within compounds of varying complexities, reinforcing your understanding of elemental compositions.
5. Double-Check Your Counting
Precision is paramount in the realm of counting atoms. After determining the total count of each element within a compound, it's beneficial to double-check your counting to ensure accuracy. Verifying the atom count provides an additional layer of assurance, minimizing the likelihood of errors and enhancing your proficiency in deciphering chemical formulas.
By incorporating these tips into your approach to counting atoms in chemical formulas, you elevate your ability to unravel the elemental compositions of diverse compounds with precision and confidence. As you navigate the captivating realm of chemistry, these strategies serve as invaluable tools, empowering you to decode the language of chemical formulas and gain profound insights into the building blocks of matter.
Conclusion
In conclusion, mastering the art of counting atoms in chemical formulas is a foundational skill that empowers aspiring chemists to unravel the intricate compositions of diverse compounds, gaining profound insights into the elemental building blocks of matter. Through our exploration, we have delved into the significance of chemical formulas as the symbolic language of chemistry, encapsulating vital information about the elements and their respective quantities within compounds. By understanding the basics of counting atoms, including the identification of elements, interpretation of subscripts, and navigation of grouping mechanisms and polyatomic ions, we have equipped ourselves with the proficiency to decipher and count atoms with precision and clarity.
The journey through practice problems has fortified our skills, enabling us to navigate through simple and complex compounds, unraveling their elemental compositions with confidence and accuracy. By engaging with diverse examples and honing our atom counting abilities, we have laid a solid foundation for comprehending the intricate structures and behaviors of chemical compounds, setting the stage for further explorations in the captivating realm of chemistry.
As we reflect on our exploration of counting atoms, it's evident that this foundational skill serves as a gateway to understanding the properties, behaviors, and interactions of substances, guiding us in our quest to unravel the mysteries of matter. The tips provided for counting atoms offer valuable strategies to enhance our proficiency, ensuring that we approach chemical formulas with attention to detail and precision.
Ultimately, the mastery of counting atoms in chemical formulas empowers us to decipher the language of compounds, unveiling the elemental compositions that form the very fabric of our world. This skill not only fosters a deeper appreciation for the complexities of matter but also lays the groundwork for further advancements in the realms of chemistry and beyond.
As we embrace the illuminating world of chemistry, let us carry forth our newfound proficiency in counting atoms, allowing it to serve as a guiding light in our scientific endeavors. With each compound we decipher and each atom we count, we deepen our understanding of the elements that shape the world around us, embarking on a journey of discovery and enlightenment in the captivating realm of chemistry.