Home>Science>Discover The Set Of Elements With Identical Chemical Properties!

Science
Discover The Set Of Elements With Identical Chemical Properties!
Published: January 20, 2024
Explore the fascinating world of science and uncover the set of elements with identical chemical properties. Dive into the wonders of chemistry today!
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Regretless.com, at no extra cost. Learn more)
Table of Contents
Introduction
The world of chemistry is a fascinating realm where elements and compounds interact in mesmerizing ways, giving rise to the myriad substances that surround us. At the heart of this intricate dance are the chemical properties of elements, which define their behavior and interactions with other substances. Understanding these properties is crucial for unraveling the mysteries of matter and harnessing its potential for various applications.
Chemical properties encompass a diverse array of characteristics that dictate how an element will react under specific conditions. These properties include reactivity, flammability, oxidation states, and the ability to form compounds. They offer profound insights into the behavior of elements, shedding light on their potential applications in fields ranging from medicine and agriculture to manufacturing and environmental science.
As we delve into the captivating realm of chemical properties, we will explore the pivotal role of the periodic table in organizing elements based on their properties. This iconic table serves as a treasure trove of knowledge, offering a systematic arrangement of elements that illuminates their unique chemical behaviors and patterns. By deciphering the periodic table, scientists and enthusiasts alike can unravel the intricate tapestry of chemical properties that underpin the natural world.
Join us on this enlightening journey as we unravel the enigmatic world of chemical properties and discover the captivating patterns that govern the behavior of elements. From the electrifying reactivity of alkali metals to the noble stability of inert gases, the periodic table unveils a rich tapestry of chemical marvels waiting to be explored. Let's embark on this adventure and unlock the secrets of the elements with identical chemical properties, unraveling the captivating mysteries that shape our world.
Read more: Element TVs: The Ultimate Review
Definition of Chemical Properties
Chemical properties are the intrinsic characteristics of an element that determine how it behaves in chemical reactions or interactions with other substances. Unlike physical properties, which can be observed without altering the substance's composition, chemical properties manifest during chemical reactions, leading to the formation of new substances. These properties provide valuable insights into an element's reactivity, stability, and affinity for bonding with other elements.
Reactivity is a fundamental chemical property that describes an element's tendency to undergo chemical reactions. Elements with high reactivity readily participate in reactions, often exhibiting vigorous behaviors such as combustion or rapid oxidation. In contrast, elements with low reactivity display inert behavior, resisting participation in chemical reactions under normal conditions.
Another crucial chemical property is electronegativity, which influences an element's ability to attract and bond with electrons. Elements with high electronegativity, such as fluorine and oxygen, tend to form strong bonds with other elements, contributing to the stability of compounds they participate in. Conversely, elements with low electronegativity, like alkali metals, readily donate electrons, leading to the formation of ionic compounds.
Furthermore, oxidation states, or oxidation numbers, are vital chemical properties that indicate an element's ability to gain or lose electrons in compounds. These states play a pivotal role in redox reactions, where electrons are transferred between elements, driving chemical changes. Understanding an element's oxidation states provides crucial insights into its role in various chemical processes and the compounds it can form.
Additionally, the ability of an element to form compounds with specific properties is a defining chemical characteristic. For instance, carbon's unique property of forming stable covalent bonds gives rise to a diverse array of organic compounds, underpinning the foundation of life and numerous industrial applications.
By comprehending the intricate web of chemical properties, scientists can predict and manipulate the behavior of elements, enabling the development of innovative materials, pharmaceuticals, and technologies. These properties serve as the building blocks of chemical knowledge, empowering researchers to unravel the complexities of matter and harness its potential for diverse applications.
Understanding chemical properties is pivotal for various scientific disciplines, from pharmaceutical research and materials science to environmental remediation and energy production. By unraveling the enigmatic nature of chemical properties, scientists pave the way for groundbreaking discoveries and innovations that shape our world.
Periodic Table and Chemical Properties
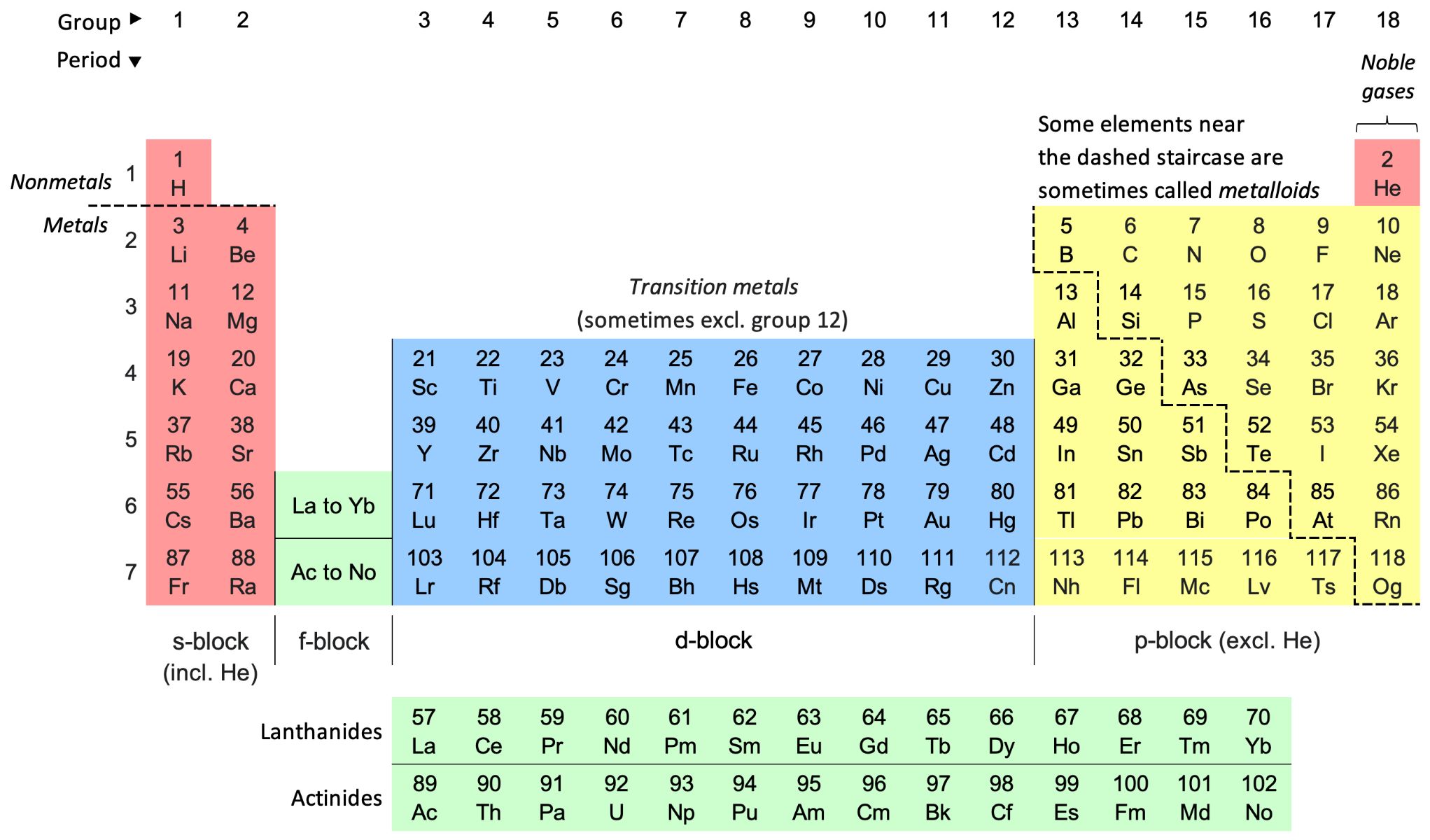
The periodic table stands as a monumental achievement in the realm of science, providing a systematic framework for understanding the chemical properties of elements. This iconic table, initially devised by Dmitri Mendeleev and subsequently refined over the years, organizes elements based on their atomic number, electron configuration, and recurring chemical properties. By arranging elements in rows and columns, the periodic table unveils captivating patterns that illuminate the intrinsic chemical behaviors of elements.
The rows of the periodic table, known as periods, showcase the gradual progression of atomic numbers and electron configurations. As elements traverse across a period, their chemical properties exhibit discernible trends, reflecting the shifting arrangement of electrons within their atomic structure. This progression offers profound insights into the variations in reactivity, electronegativity, and bonding tendencies among elements as their atomic numbers increase.
Moreover, the columns of the periodic table, referred to as groups or families, encapsulate elements with similar chemical properties. Elements within the same group share common characteristics, owing to their identical valence electron configurations. This shared electron configuration imparts analogous reactivity and bonding behaviors, leading to the emergence of distinctive chemical trends within each group.
The periodic table's organization enables scientists to discern recurring patterns in the chemical properties of elements, facilitating predictions about their behavior in chemical reactions and their potential applications in various fields. For instance, the alkali metals in Group 1 exhibit a remarkable propensity for reacting with water, showcasing a trend of increasing reactivity down the group. In contrast, the noble gases in Group 18 display inert behavior, underscoring their stability and reluctance to engage in chemical reactions.
By deciphering the periodic table, researchers gain profound insights into the underlying principles that govern the chemical properties of elements. This knowledge serves as a cornerstone for diverse scientific endeavors, from designing innovative materials and catalysts to elucidating the behavior of elements in biological systems. The periodic table's role in elucidating the intricate tapestry of chemical properties underscores its enduring significance as a foundational tool in the pursuit of scientific knowledge and discovery.
Groups with Identical Chemical Properties
The periodic table categorizes elements into groups, each of which exhibits distinct chemical behaviors stemming from their shared electron configurations. These groups, also known as families, play a pivotal role in unveiling the captivating patterns that govern the chemical properties of elements. By exploring the defining characteristics of select groups, we can unravel the mesmerizing tapestry of identical chemical properties that underpin the behavior of elements.
Alkali Metals (Group 1)
The alkali metals, including lithium, sodium, and potassium, form Group 1 of the periodic table. These elements boast a singular valence electron, conferring them with remarkable reactivity. They readily donate this lone electron, displaying a fervent affinity for forming ionic compounds with non-metals. This distinctive behavior manifests in their vigorous reactions with water, producing hydrogen gas and alkaline hydroxides. As we descend the group, reactivity intensifies, culminating in the explosive reaction of cesium with water. The alkali metals' propensity for rapid oxidation underscores their pivotal role in numerous industrial processes and energy storage technologies.
Alkaline Earth Metals (Group 2)
Group 2 comprises the alkaline earth metals, encompassing beryllium, magnesium, and calcium, among others. With two valence electrons, these elements exhibit notable reactivity, albeit less pronounced than their Group 1 counterparts. They readily form ionic compounds, showcasing a penchant for bonding with non-metals to create diverse salts and minerals. The alkaline earth metals play pivotal roles in various applications, from structural alloys and biomedical implants to agricultural fertilizers and environmental remediation technologies.
Halogens (Group 17)
The halogens, including fluorine, chlorine, and iodine, constitute Group 17, showcasing distinct chemical properties rooted in their seven valence electrons. These elements exhibit high electronegativity, displaying a fervent penchant for forming compounds with metals and non-metals. Their reactivity diminishes as we move down the group, with fluorine exhibiting vigorous behavior, while iodine displays a more subdued nature. The halogens' ability to form acidic compounds and participate in redox reactions underscores their pivotal roles in diverse industrial processes, from water treatment to pharmaceutical synthesis.
Noble Gases (Group 18)
Group 18, the noble gases, represents a cohort of elements celebrated for their inert nature. With complete valence electron shells, these elements exhibit minimal reactivity, refraining from forming compounds under standard conditions. Their stability and reluctance to engage in chemical reactions underpin their pivotal roles in various applications, from inert atmospheres in industrial processes to lighting and electronics.
The periodic table's organization into groups with identical chemical properties unveils a captivating tapestry of elemental behaviors, offering profound insights into the diverse applications and implications of these shared properties. By delving into the captivating world of these elemental cohorts, scientists unlock the intricate secrets that shape the behavior of elements and pave the way for groundbreaking innovations across myriad scientific disciplines.
Examples of Elements with Identical Chemical Properties
The periodic table serves as a captivating tapestry of elemental behaviors, showcasing distinct groups of elements with identical chemical properties. These elemental cohorts offer profound insights into the captivating patterns that govern the behavior of elements, unveiling a rich array of applications and implications. Let's delve into the intriguing world of select groups and unravel the mesmerizing examples of elements with identical chemical properties.
Alkali Metals (Group 1)
The alkali metals, including lithium, sodium, and potassium, exemplify a group with identical chemical properties rooted in their singular valence electron. This shared characteristic imparts remarkable reactivity, leading to analogous behaviors in chemical reactions. For instance, all alkali metals exhibit a fervent affinity for donating their lone electron, leading to the formation of ionic compounds with non-metals. This shared trait manifests in their vigorous reactions with water, culminating in the production of hydrogen gas and alkaline hydroxides. As we traverse the group, reactivity intensifies, showcasing a captivating trend of increasing vigor in chemical interactions. The alkali metals' shared chemical properties underpin their pivotal roles in diverse industrial processes and energy storage technologies, offering a captivating glimpse into the cohesive behaviors of these elements.
Halogens (Group 17)
Group 17, the halogens, presents a captivating ensemble of elements with identical chemical properties stemming from their seven valence electrons. This shared characteristic bestows high electronegativity upon the halogens, leading to analogous behaviors in their interactions with other elements. For example, all halogens exhibit a fervent penchant for forming compounds with metals and non-metals, showcasing a shared reactivity that diminishes as we descend the group. Fluorine, the most reactive halogen, displays vigorous behavior, while iodine, positioned at the group's lower end, exhibits a more subdued nature. The halogens' shared chemical properties find diverse applications, from water treatment and disinfection to pharmaceutical synthesis, underscoring the profound implications of their cohesive behaviors.
Noble Gases (Group 18)
Group 18, the noble gases, represents a captivating cohort of elements celebrated for their inert nature. With complete valence electron shells, these elements share the identical property of minimal reactivity, refraining from forming compounds under standard conditions. This cohesive behavior underscores their pivotal roles in various applications, from providing inert atmospheres in industrial processes to serving as essential components in lighting and electronics. The noble gases' shared chemical properties offer a captivating glimpse into the cohesive behaviors of these elements, paving the way for groundbreaking innovations across diverse scientific disciplines.
In unraveling the mesmerizing examples of elements with identical chemical properties, we gain profound insights into the captivating patterns that govern the behavior of elements. These shared properties underscore the cohesive nature of elemental cohorts, offering a captivating tapestry of behaviors that shape the foundations of chemical knowledge and innovation.
Conclusion
The captivating journey through the realm of chemical properties has unveiled a rich tapestry of elemental behaviors, showcasing the intricate patterns that govern the behavior of elements. From the electrifying reactivity of alkali metals to the noble stability of inert gases, the periodic table stands as a testament to the profound insights it offers into the cohesive behaviors of elemental cohorts.
By delving into the captivating world of chemical properties, we have gained a deeper appreciation for the pivotal role of the periodic table in organizing elements based on their shared characteristics. The arrangement of elements into groups and periods has unveiled mesmerizing trends in reactivity, electronegativity, and bonding tendencies, offering profound insights into the diverse applications and implications of these shared properties.
Furthermore, the exploration of select groups, such as the alkali metals, alkaline earth metals, halogens, and noble gases, has illuminated the captivating examples of elements with identical chemical properties. These elemental cohorts, characterized by shared valence electron configurations, exhibit cohesive behaviors that underpin their pivotal roles in diverse industrial processes, technological innovations, and scientific endeavors.
As we conclude this enlightening journey, the significance of understanding chemical properties becomes abundantly clear. These properties serve as the building blocks of chemical knowledge, empowering researchers to predict and manipulate the behavior of elements, enabling the development of innovative materials, pharmaceuticals, and technologies. The cohesive behaviors exhibited by elemental cohorts underscore the enduring significance of the periodic table as a foundational tool in the pursuit of scientific knowledge and discovery.
In essence, the exploration of chemical properties transcends the confines of the laboratory, permeating diverse facets of our lives. From the materials that shape our built environment to the pharmaceuticals that enhance our well-being, the cohesive behaviors of elements underpin countless innovations and advancements. By unraveling the mesmerizing tapestry of chemical properties, scientists pave the way for groundbreaking discoveries and innovations that shape our world, offering a testament to the enduring allure and significance of this captivating realm of scientific inquiry.