Home>Science>Unveiling The Secrets: NaBH4 Vs LiAlH4 – Which Wins The Battle?

Science
Unveiling The Secrets: NaBH4 Vs LiAlH4 – Which Wins The Battle?
Published: January 14, 2024
Uncover the scientific showdown between NaBH4 and LiAlH4 to determine the ultimate victor in this chemical battle. Delve into the secrets of these powerful reagents and their impact on the world of science.
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Regretless.com, at no extra cost. Learn more)
Table of Contents
Introduction
In the realm of science, chemical compounds play a pivotal role in various applications, from pharmaceuticals to materials science. Among these compounds, sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) stand out as significant reagents in organic chemistry. Their unique properties and reactivity make them indispensable in the synthesis of organic compounds, but understanding their differences and applications is crucial for harnessing their potential effectively.
As we delve into the world of chemical reagents, the battle between NaBH4 and LiAlH4 unfolds, piquing the curiosity of scientists and enthusiasts alike. These compounds possess distinct characteristics that set them apart, and exploring their individual attributes unveils a fascinating narrative of chemical reactivity and practical applications.
Let's embark on a journey to unravel the secrets of NaBH4 and LiAlH4, shedding light on their chemical properties, reactivity, and diverse applications. By delving into their unique features, we can gain a deeper understanding of their roles in organic synthesis and the broader scientific landscape.
What are NaBH4 and LiAlH4?
Sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) are powerful chemical reagents that play pivotal roles in organic synthesis. These compounds are renowned for their ability to facilitate a wide range of chemical reactions, making them indispensable in the realm of organic chemistry.
Sodium Borohydride (NaBH4)
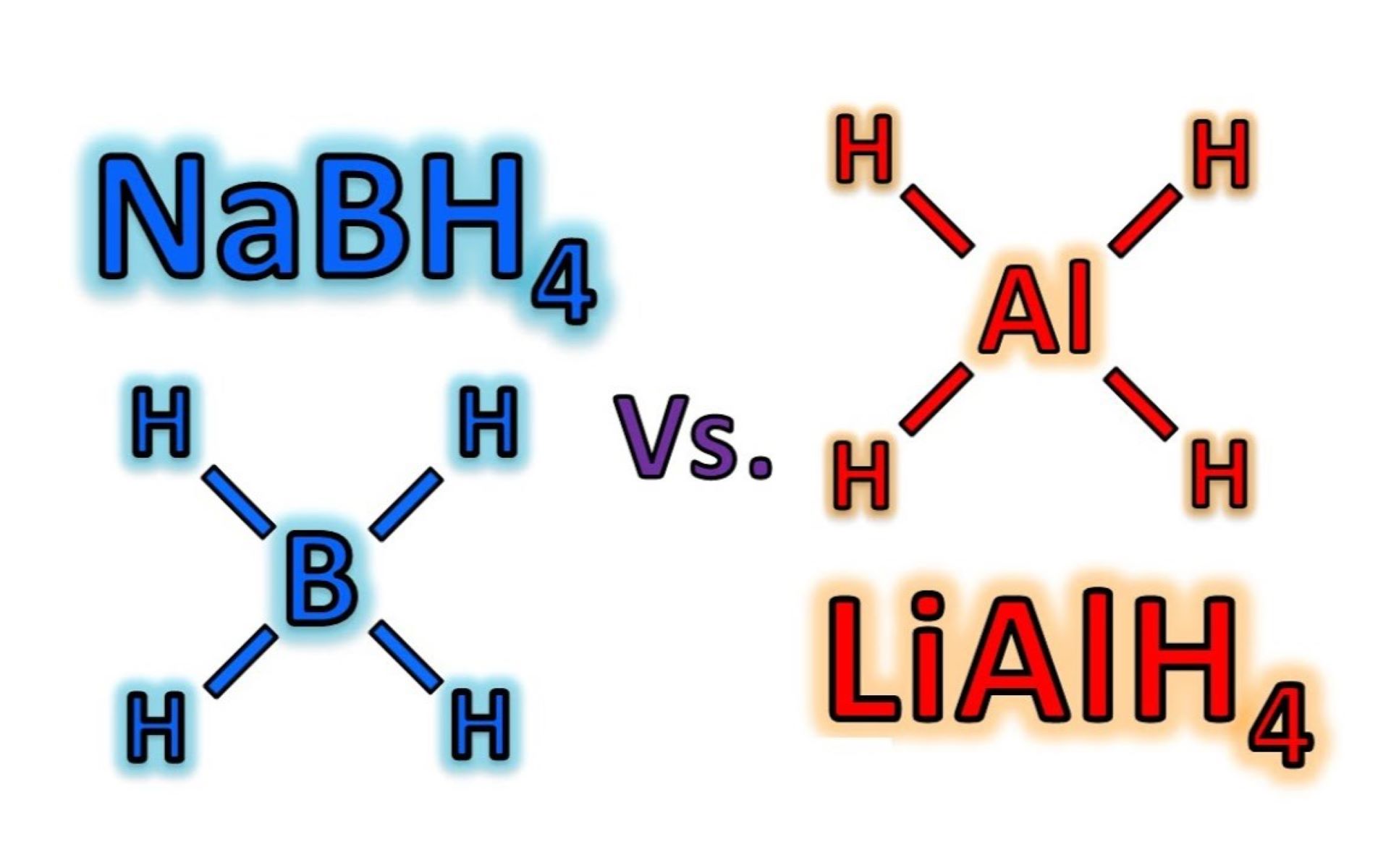
Sodium borohydride, with the chemical formula NaBH4, is a versatile and widely used reagent in organic synthesis. It is a white, crystalline solid that is highly soluble in water, giving it the ability to undergo reactions in aqueous solutions. NaBH4 is a mild reducing agent, making it particularly useful in the reduction of carbonyl compounds, such as aldehydes and ketones, to their respective alcohols. This reactivity stems from the hydride ion (H-) donated by NaBH4, which serves as the reducing agent in these transformations. Additionally, NaBH4 is known for its relatively high stability, allowing for safe handling and storage, which further contributes to its widespread use in laboratory settings.
Lithium Aluminum Hydride (LiAlH4)
Lithium aluminum hydride, represented by the chemical formula LiAlH4, is a potent reducing agent with a broad scope of reactivity. Unlike NaBH4, LiAlH4 is not water-soluble and is typically used in non-aqueous solvents. This compound is highly reactive due to the presence of four hydride ions, which enables it to reduce a wide range of functional groups, including carboxylic acids, esters, and even some types of halides. LiAlH4 is known for its pyrophoric nature, requiring careful handling and storage to prevent accidental ignition upon exposure to air.
In summary, NaBH4 and LiAlH4 are both essential reagents in organic chemistry, each with its unique set of properties and reactivity. While NaBH4 is prized for its mild and selective reduction capabilities, LiAlH4 stands out as a potent and versatile reducing agent. Understanding the distinct characteristics of these compounds is crucial for harnessing their potential in organic synthesis and other chemical applications.
Chemical Properties
Sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) exhibit distinct chemical properties that underpin their reactivity and utility in organic synthesis. Understanding these properties is essential for leveraging the unique capabilities of these compounds in various chemical transformations.
Sodium Borohydride (NaBH4)
NaBH4 is characterized by its mild reducing properties, attributed to the hydride ion (H-) it donates in chemical reactions. This compound is stable in aqueous solutions, allowing for efficient reduction of carbonyl compounds, such as aldehydes and ketones, to their respective alcohols. The selective nature of NaBH4's reduction reactions makes it a valuable tool in organic synthesis, enabling precise control over the functional group transformations. Additionally, NaBH4 exhibits high solubility in water, enhancing its compatibility with aqueous reaction conditions and facilitating its use in a wide range of synthetic processes.
Lithium Aluminum Hydride (LiAlH4)
In contrast, lithium aluminum hydride (LiAlH4) boasts potent reducing capabilities owing to the presence of four hydride ions. This compound is highly reactive and can effectively reduce various functional groups, including carboxylic acids, esters, and certain types of halides. Unlike NaBH4, LiAlH4 is not water-soluble and is typically used in non-aqueous solvents to harness its powerful reducing properties. However, the pyrophoric nature of LiAlH4 demands cautious handling and storage to prevent accidental ignition, adding an extra layer of complexity to its use in laboratory settings.
Distinctive Attributes
Both NaBH4 and LiAlH4 play crucial roles in organic synthesis, albeit with distinct chemical properties that dictate their reactivity and applications. NaBH4's mild and selective nature makes it well-suited for specific reduction reactions, while LiAlH4's potent reducing capabilities enable a broader scope of functional group transformations. Understanding the chemical properties of these compounds empowers chemists to make informed decisions when selecting reagents for synthetic processes, ultimately driving the advancement of organic chemistry and related fields.
In essence, the distinct chemical properties of NaBH4 and LiAlH4 underscore their significance as versatile reagents in organic synthesis, laying the foundation for diverse chemical transformations and scientific advancements.
Reactivity
The reactivity of sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) is a defining aspect of their utility in organic synthesis and chemical transformations. Understanding the distinct reactivity of these compounds is essential for leveraging their capabilities in diverse synthetic processes.
Sodium Borohydride (NaBH4)
NaBH4 exhibits mild and selective reactivity as a reducing agent, primarily attributed to the hydride ion (H-) it donates in chemical reactions. This unique reactivity enables NaBH4 to effectively reduce a specific set of functional groups, particularly carbonyl compounds such as aldehydes and ketones. The selective nature of NaBH4's reduction reactions allows for precise control over the transformation of carbonyl groups into their respective alcohols, making it a valuable tool in organic synthesis. Additionally, NaBH4's high solubility in water enhances its reactivity in aqueous solutions, facilitating its use in a wide range of synthetic processes. This selective reactivity contributes to the controlled and efficient conversion of targeted functional groups, underscoring the significance of NaBH4 in organic chemistry.
Lithium Aluminum Hydride (LiAlH4)
In contrast, lithium aluminum hydride (LiAlH4) is characterized by its potent and broad reactivity as a reducing agent. The presence of four hydride ions in LiAlH4 enables it to effectively reduce a wide range of functional groups, including carboxylic acids, esters, and certain types of halides. This broad reactivity makes LiAlH4 a versatile tool in organic synthesis, allowing for the transformation of diverse functional groups with high efficiency. However, the reactivity of LiAlH4 comes with the caveat of its pyrophoric nature, necessitating careful handling and storage to prevent accidental ignition upon exposure to air. Despite this challenge, the potent reactivity of LiAlH4 makes it an indispensable reagent for complex chemical transformations in organic synthesis.
In essence, the reactivity of NaBH4 and LiAlH4 embodies their distinct roles as reducing agents in organic chemistry. NaBH4's selective reactivity enables precise control over specific functional group transformations, while LiAlH4's broad reactivity empowers the efficient reduction of diverse functional groups. Understanding and harnessing the unique reactivity of these compounds is integral to advancing the field of organic synthesis and driving scientific innovation.
Applications
The versatile nature of sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) extends beyond their distinct chemical properties and reactivity, finding diverse applications in organic synthesis and related fields. These compounds serve as indispensable reagents in a wide range of chemical transformations, contributing to the advancement of pharmaceuticals, materials science, and various industrial processes.
Sodium Borohydride (NaBH4)
NaBH4's mild and selective reducing properties make it a valuable tool in organic synthesis, particularly in the pharmaceutical industry. Its ability to efficiently reduce carbonyl compounds, such as aldehydes and ketones, to their respective alcohols is pivotal in the synthesis of pharmaceutical intermediates and active compounds. Additionally, NaBH4 plays a crucial role in the production of fine chemicals and specialty materials, where controlled reduction reactions are essential for achieving desired chemical structures and properties.
Furthermore, NaBH4 finds applications in the development of sustainable energy technologies, particularly in the synthesis of hydrogen storage materials. Its reactivity in aqueous solutions enables the efficient generation of hydrogen gas, contributing to research efforts aimed at advancing hydrogen fuel technologies and renewable energy systems.
Lithium Aluminum Hydride (LiAlH4)
The potent reducing capabilities of LiAlH4 render it indispensable in diverse chemical transformations, with notable applications in the synthesis of pharmaceuticals and agrochemicals. Its ability to efficiently reduce a wide range of functional groups, including carboxylic acids and esters, is instrumental in the production of complex organic molecules with therapeutic and agricultural significance.
Moreover, LiAlH4 plays a crucial role in materials science, particularly in the synthesis of advanced materials with tailored properties. Its broad reactivity enables the reduction of various functional groups, facilitating the production of specialty polymers, catalysts, and electronic materials. Additionally, LiAlH4 contributes to the development of novel materials for energy storage and conversion, where precise control over chemical transformations is paramount for enhancing performance and efficiency.
In summary, the applications of NaBH4 and LiAlH4 span across diverse sectors, encompassing pharmaceutical synthesis, materials science, sustainable energy research, and industrial processes. The unique reactivity and selective or broad reducing capabilities of these compounds underpin their pivotal roles in advancing scientific innovation and addressing complex challenges across various domains.
This comprehensive exploration of the applications of NaBH4 and LiAlH4 underscores their significance as versatile reagents with far-reaching implications in organic synthesis and beyond.
Safety Considerations
When working with sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4), prioritizing safety is paramount due to the reactive nature of these compounds. Understanding and adhering to stringent safety protocols is essential to mitigate potential hazards and ensure a secure laboratory environment.
Sodium Borohydride (NaBH4)
NaBH4, while generally considered a mild and stable reagent, requires careful handling to prevent unintended reactions and exposure. As a water-soluble compound, it is crucial to avoid contact with moisture, as this can lead to the release of flammable hydrogen gas. Additionally, NaBH4 poses a risk of skin and eye irritation upon direct contact, necessitating the use of personal protective equipment, including gloves, goggles, and lab coats, when handling the compound.
Furthermore, NaBH4 should be stored in a cool, dry place away from incompatible substances to prevent potential reactions. In the event of a spill or release, prompt action must be taken to contain and neutralize the compound using appropriate absorbent materials and chemical neutralizers. Proper ventilation in the laboratory is essential to prevent the accumulation of hydrogen gas and mitigate the risk of flammability.
Lithium Aluminum Hydride (LiAlH4)
LiAlH4 presents unique safety challenges due to its pyrophoric nature and reactivity with moisture and air. Special precautions must be taken to prevent accidental ignition and exposure. Handling LiAlH4 requires the use of specialized equipment, such as air-free techniques and inert atmospheres, to minimize the risk of fire and ensure safe manipulation.
Storage of LiAlH4 necessitates the use of air-tight containers in a controlled environment to prevent moisture ingress and potential reactivity. In the event of a fire or spill involving LiAlH4, appropriate fire suppression measures and chemical neutralization protocols must be followed to mitigate the associated hazards effectively.
Overall Safety Precautions
In addition to specific safety considerations for NaBH4 and LiAlH4, general laboratory safety practices, including proper waste disposal, emergency response procedures, and regular safety training, are vital for maintaining a secure working environment. Adequate personal protective equipment, comprehensive risk assessments, and meticulous adherence to standard operating procedures are indispensable in minimizing the potential risks associated with these compounds.
By prioritizing safety considerations and implementing robust safety measures, researchers and laboratory personnel can harness the potential of NaBH4 and LiAlH4 while ensuring a secure and controlled working environment.
This detailed overview of safety considerations underscores the critical importance of implementing rigorous safety protocols when working with NaBH4 and LiAlH4, contributing to a culture of safety and responsibility in laboratory settings.
Conclusion
In conclusion, the comparison between sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) unveils a fascinating narrative of contrasting reactivity, chemical properties, and diverse applications in organic synthesis and related fields. NaBH4, with its mild and selective reducing capabilities, serves as a valuable tool for controlled reduction reactions, particularly in the pharmaceutical industry and sustainable energy research. Its compatibility with aqueous solutions and high solubility in water further enhances its utility in a wide range of synthetic processes.
On the other hand, LiAlH4 emerges as a potent and versatile reducing agent, offering broad reactivity that extends to the efficient reduction of various functional groups, making it indispensable in pharmaceutical synthesis, materials science, and the development of advanced materials for energy storage and conversion.
The distinct chemical properties and reactivity of NaBH4 and LiAlH4 underscore their significance as versatile reagents that underpin scientific innovation and address complex challenges across diverse sectors. However, it is crucial to emphasize the paramount importance of safety considerations when working with these compounds, given their reactive nature and associated hazards. Adhering to stringent safety protocols and implementing robust safety measures is essential to ensure a secure laboratory environment and mitigate potential risks.
Overall, the exploration of NaBH4 and LiAlH4 highlights their pivotal roles in advancing organic synthesis, materials science, and sustainable energy research. Their unique capabilities contribute to the development of novel pharmaceuticals, specialty materials, and sustainable energy technologies, shaping the landscape of scientific and industrial advancements. By understanding the distinct attributes of NaBH4 and LiAlH4, scientists and researchers can harness their potential effectively, driving the progress of organic chemistry and related fields.
In essence, the battle between NaBH4 and LiAlH4 transcends a mere comparison of reagents; it represents a quest for innovation, safety, and transformative impact in the realm of chemical synthesis and scientific exploration.