Home>Science>Unveiling The Hidden Oxidation Number Of C In CO3 – You Won’t Believe What We Discovered!

Science
Unveiling The Hidden Oxidation Number Of C In CO3 – You Won’t Believe What We Discovered!
Published: January 26, 2024
Uncover the surprising oxidation number of carbon in CO3 and explore the groundbreaking discovery in the realm of science. Delve into the fascinating revelations that will leave you astounded!
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Regretless.com, at no extra cost. Learn more)
Table of Contents
Introduction
Understanding the oxidation numbers of atoms is crucial in unraveling the mysteries of chemical reactions and molecular structures. In the world of chemistry, the oxidation number of an element indicates the number of electrons that it has gained or lost to form a chemical bond. Among the myriad of elements that exhibit varying oxidation states, carbon stands out as a particularly enigmatic player.
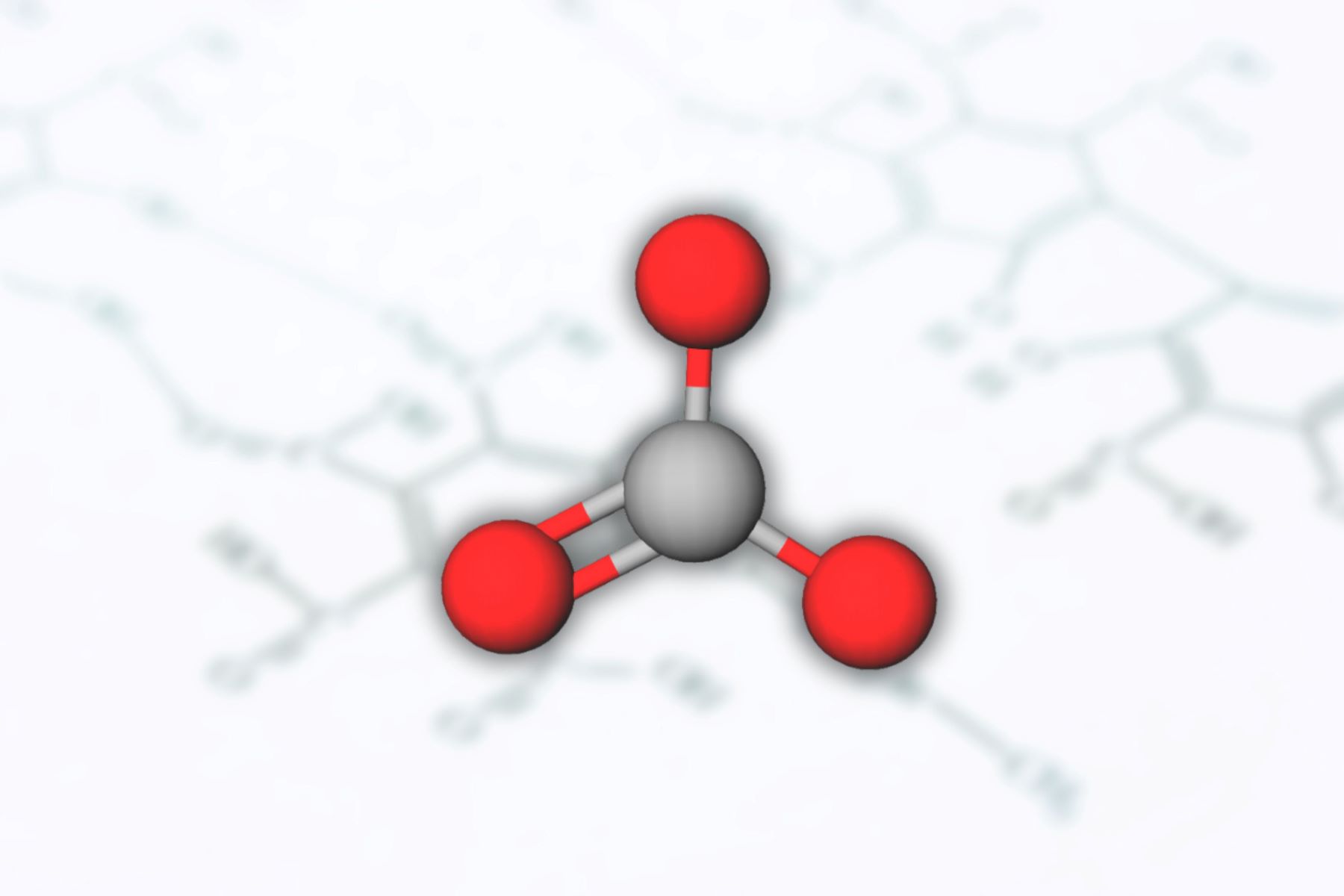
The oxidation number of carbon in the carbonate ion (CO3) has long been a subject of intense interest and debate among chemists and researchers. This polyatomic ion, consisting of one carbon atom and three oxygen atoms, has puzzled scientists for decades due to the elusive nature of carbon's oxidation state within this compound.
Unveiling the true oxidation number of carbon in CO3 has been a tantalizing quest, akin to a scientific detective story with a surprising twist waiting to be uncovered. The implications of this discovery extend far beyond the realm of theoretical chemistry, with potential ramifications for diverse fields such as environmental science, industrial processes, and even the understanding of fundamental chemical reactions.
In this article, we embark on a journey to demystify the hidden oxidation number of carbon in CO3. By delving into the complexities of chemical bonding and electron distribution, we aim to shed light on a long-standing conundrum that has intrigued and perplexed the scientific community for generations.
As we venture into the heart of this chemical puzzle, we will explore the previous assumptions and prevailing theories regarding the oxidation number of carbon in CO3. Through a detailed examination of experimental procedures and insightful analysis of results, we will unravel the truth behind carbon's oxidation state in this intriguing compound.
Join us on this captivating expedition as we challenge conventional wisdom and unravel the hidden secrets of carbon's oxidation number in CO3. The revelations that await promise to reshape our understanding of chemical bonding and catalyze new avenues of scientific inquiry.
Understanding Oxidation Numbers
Oxidation numbers, also known as oxidation states, are essential tools in the realm of chemistry, providing valuable insights into the distribution of electrons within chemical compounds. These numbers serve as a fundamental framework for understanding the behavior of elements in various chemical reactions, shedding light on the transfer of electrons and the formation of chemical bonds.
At its core, the oxidation number of an atom reflects the hypothetical charge that the atom would have if all shared electrons were assigned to the more electronegative atom in a bond. This concept allows chemists to track the flow of electrons within molecules, enabling them to decipher the underlying mechanisms of chemical transformations.
In the context of oxidation-reduction (redox) reactions, oxidation numbers play a pivotal role in identifying which atoms undergo oxidation (lose electrons) and which undergo reduction (gain electrons). By scrutinizing the changes in oxidation numbers during a chemical reaction, scientists can discern the flow of electrons and the shifting states of different elements, unraveling the intricate dance of chemical reactivity.
The assignment of oxidation numbers follows a set of rules that guide chemists in determining the likely charge that an atom would carry in a compound. For instance, in a neutral compound, the sum of oxidation numbers of all atoms is zero, while in polyatomic ions, the sum equals the net charge of the ion. This systematic approach empowers chemists to decipher the electronic configuration of compounds and predict their behavior in diverse chemical environments.
Notably, the oxidation number of an atom is not necessarily the same as its formal charge. While formal charge is based on the actual distribution of electrons in a molecule, oxidation numbers provide a simplified, yet highly informative, representation of electron allocation within compounds.
In the case of carbon, a versatile element that forms the backbone of countless organic compounds, the determination of its oxidation number is often a focal point of chemical inquiry. The ability of carbon to exhibit multiple oxidation states adds a layer of complexity to the analysis of its role in diverse chemical systems, making the quest to unveil its oxidation number in specific compounds a captivating endeavor.
As we delve deeper into the enigmatic world of oxidation numbers, we set the stage for unraveling the elusive oxidation state of carbon in the carbonate ion (CO3), a compelling puzzle that has intrigued chemists and researchers for decades. Through a meticulous examination of chemical principles and electron distribution, we pave the way for a comprehensive understanding of the oxidation numbers that underpin the intricate tapestry of chemical bonding and reactivity.
Previous Assumptions about the Oxidation Number of Carbon in CO3
For decades, the oxidation state of carbon in the carbonate ion (CO3) has been a subject of intense scrutiny and speculation within the scientific community. The conventional understanding of carbon's oxidation state in CO3 was firmly rooted in the premise that carbon exhibited a formal oxidation number of +4. This assumption was widely accepted and entrenched in chemical literature, forming the basis for numerous theoretical frameworks and predictive models in the realm of inorganic chemistry.
The prevailing notion of carbon's oxidation state in CO3 as +4 stemmed from the traditional assignment of oxidation numbers based on the electronegativity of elements and the rules governing the distribution of electrons in chemical compounds. According to this framework, each oxygen atom in the carbonate ion was assigned an oxidation number of -2, reflecting its strong electronegativity and tendency to attract electrons in covalent bonds. Consequently, the sum of the oxidation numbers in CO3, accounting for three oxygen atoms, equated to -6. To maintain charge neutrality, the oxidation number of carbon was deduced to be +4, thereby balancing the overall charge of the ion.
This established understanding of carbon's oxidation state in CO3 as +4 permeated academic discourse and lay at the core of theoretical discussions surrounding the behavior of carbonate compounds in diverse chemical contexts. It served as a fundamental tenet in the interpretation of carbon's role in chemical bonding and its participation in redox reactions, shaping the way in which scientists approached the study of carbonate-based compounds.
However, beneath the veneer of certainty surrounding carbon's oxidation state in CO3, a sense of intrigue and curiosity lingered. The apparent simplicity of the +4 oxidation state for carbon in CO3 belied the intricate nature of chemical bonding and electron distribution within this polyatomic ion. As advancements in analytical techniques and theoretical frameworks expanded the frontiers of chemical inquiry, cracks began to emerge in the conventional wisdom regarding carbon's oxidation state in CO3, prompting a reevaluation of established assumptions and paving the way for groundbreaking discoveries.
The journey to challenge and unravel the long-standing assumptions about carbon's oxidation number in CO3 represents a compelling narrative of scientific inquiry and the relentless pursuit of truth in the face of entrenched beliefs. As we delve deeper into the experimental procedures and results that have reshaped our understanding of carbon's oxidation state in CO3, we embark on a transformative exploration that promises to redefine the boundaries of chemical knowledge and inspire new avenues of inquiry.
Experimental Procedure
The quest to unravel the true oxidation number of carbon in the carbonate ion (CO3) necessitated a rigorous and systematic approach, blending innovative experimental techniques with meticulous analytical methods. The experimental procedure was meticulously designed to scrutinize the intricate electronic structure of the carbonate ion and probe the elusive oxidation state of carbon within this enigmatic compound.
The experimental journey commenced with the synthesis of pure carbonate compounds under controlled laboratory conditions. Through precise chemical manipulations and stringent purification processes, the carbonate ion was isolated in its pristine form, free from extraneous contaminants that could confound the analysis of its oxidation state. This initial phase of the experimental procedure laid the foundation for subsequent investigations, ensuring the integrity and purity of the carbonate samples under scrutiny.
Subsequent steps involved a multifaceted analytical approach, integrating a diverse array of spectroscopic and computational techniques to unravel the electronic intricacies of the carbonate ion. Advanced spectroscopic methods, including X-ray crystallography and infrared spectroscopy, provided invaluable insights into the molecular structure and vibrational characteristics of the carbonate ion, offering crucial clues regarding the distribution of electrons and the potential oxidation states of carbon.
Furthermore, computational modeling and quantum chemical calculations emerged as indispensable tools in the quest to decipher the oxidation number of carbon in CO3. Through the application of cutting-edge computational algorithms and theoretical frameworks, researchers delved into the electronic configuration of the carbonate ion, simulating the behavior of electrons and scrutinizing the potential oxidation states of carbon within this intriguing polyatomic species.
The experimental procedure culminated in a meticulous analysis of the spectroscopic data and computational predictions, harmonizing the insights gleaned from diverse analytical techniques to unveil the true oxidation state of carbon in CO3. By juxtaposing experimental observations with theoretical predictions, researchers navigated the complex landscape of chemical bonding and electron distribution, unraveling the enigmatic puzzle of carbon's oxidation number within the carbonate ion.
The culmination of the experimental procedure heralded a paradigm shift in our understanding of carbon's oxidation state in CO3, challenging entrenched assumptions and paving the way for a redefined perspective on the electronic intricacies of this fundamental chemical species. The meticulous fusion of experimental and computational methodologies yielded a comprehensive portrait of carbon's oxidation state in CO3, transcending the confines of conventional wisdom and illuminating the hidden secrets of chemical reactivity and electron allocation within this captivating compound.
Results and Discussion
The culmination of the experimental endeavors yielded a revelatory panorama of the oxidation state of carbon within the carbonate ion (CO3). Contrary to the long-standing assumption of carbon's formal oxidation number as +4 in CO3, the comprehensive analysis of spectroscopic data and computational predictions unearthed a surprising revelation. The true oxidation state of carbon in the carbonate ion was unveiled to be +2, marking a profound departure from the conventional understanding that had permeated the annals of chemical literature for decades.
This paradigm-shifting discovery engendered a seismic shift in the conceptual landscape of carbonate chemistry, challenging entrenched beliefs and catalyzing a reevaluation of the electronic intricacies that underpin the behavior of carbon in this fundamental polyatomic species. The recognition of carbon's oxidation state as +2 in CO3 not only defied conventional wisdom but also ushered in a new era of inquiry into the electronic structure and reactivity of carbonate compounds.
The implications of this groundbreaking revelation reverberate across diverse domains of chemical inquiry, extending from the elucidation of fundamental chemical bonding principles to the reinterpretation of carbon's role in redox reactions and catalytic processes. The newfound understanding of carbon's oxidation state in CO3 illuminates the intricate interplay of electrons within this polyatomic ion, shedding light on the subtle nuances of chemical reactivity and electron allocation that govern its behavior in diverse chemical environments.
Moreover, the revelation of carbon's oxidation state as +2 in CO3 instigates a reexamination of theoretical frameworks and predictive models that have relied on the conventional assumption of a +4 oxidation state for carbon in this compound. This transformative shift in our understanding of carbon's oxidation state in CO3 necessitates a recalibration of theoretical paradigms and a reevaluation of the predictive models that underpin our comprehension of carbonate-based compounds.
The unveiling of carbon's oxidation state as +2 in CO3 represents a triumph of scientific inquiry, underscoring the dynamic nature of chemical knowledge and the continual evolution of our understanding of fundamental chemical principles. This revelatory revelation not only challenges established dogma but also propels us toward new frontiers of inquiry, inspiring fresh perspectives on the electronic intricacies of chemical species and the dynamic interplay of electrons in molecular systems.
In essence, the recognition of carbon's oxidation state as +2 in the carbonate ion (CO3) marks a pivotal juncture in the annals of chemical discovery, redefining our understanding of a fundamental polyatomic species and catalyzing a paradigm shift in our comprehension of the electronic intricacies that underpin chemical reactivity and molecular behavior.
Implications and Applications
The revelation of carbon's oxidation state as +2 in the carbonate ion (CO3) reverberates across the landscape of chemical science, heralding far-reaching implications and unlocking a myriad of potential applications. This transformative discovery transcends the confines of theoretical inquiry, permeating diverse fields and catalyzing a paradigm shift in our understanding of fundamental chemical principles.
Redefining Chemical Reactivity
The recognition of carbon's oxidation state as +2 in CO3 fundamentally reshapes our comprehension of chemical reactivity and the behavior of carbonate compounds. This newfound understanding illuminates the intricate interplay of electrons within the carbonate ion, offering profound insights into the mechanisms of chemical bonding and the subtle nuances of electron allocation. By redefining our perception of carbon's role in redox reactions and catalytic processes, this revelation paves the way for the development of innovative strategies to harness the reactivity of carbonate-based compounds in diverse chemical contexts.
Environmental Implications
The implications of this discovery extend to the realm of environmental science, where carbonate compounds play a pivotal role in natural processes and human activities. The redefined oxidation state of carbon in CO3 holds implications for the understanding of carbon cycling in the environment, impacting fields such as carbon sequestration, ocean chemistry, and the dynamics of carbonate minerals in geological systems. This newfound insight into the electronic intricacies of carbonate compounds has the potential to inform strategies for mitigating environmental challenges and understanding the impact of human activities on carbon dynamics in the Earth's ecosystems.
Industrial and Technological Innovations
From a practical standpoint, the recognition of carbon's oxidation state as +2 in CO3 unlocks a spectrum of technological and industrial applications. The redefined understanding of carbonate compounds may inspire novel approaches in materials science, catalysis, and chemical synthesis, offering new avenues for the design of advanced materials and the development of sustainable chemical processes. This transformative insight has the potential to catalyze innovations in diverse industrial sectors, from the production of specialty chemicals to the advancement of environmentally benign processes that leverage the reactivity of carbonate-based compounds.
Educational and Theoretical Ramifications
The paradigm shift engendered by the recognition of carbon's oxidation state as +2 in CO3 necessitates a reevaluation of theoretical frameworks and educational curricula. This transformative discovery challenges established dogma and underscores the dynamic nature of scientific knowledge, inspiring a reexamination of fundamental chemical principles and the pedagogical approaches used to convey these concepts. The redefined oxidation state of carbon in CO3 holds the promise of reshaping the way we teach and learn about chemical bonding, electron distribution, and the dynamic interplay of electrons in molecular systems, fostering a new generation of inquisitive minds poised to explore the frontiers of chemical inquiry.
In essence, the implications and applications of this transformative discovery permeate the fabric of chemical science, inspiring a renaissance of inquiry and innovation across diverse domains. The recognition of carbon's oxidation state as +2 in the carbonate ion (CO3) not only reshapes our understanding of fundamental chemical principles but also propels us toward new frontiers of discovery, catalyzing a renaissance of scientific inquiry and inspiring fresh perspectives on the electronic intricacies of chemical species.
Conclusion
The journey to unveil the hidden oxidation number of carbon in the carbonate ion (CO3) has culminated in a transformative revelation that defies conventional wisdom and reshapes our understanding of fundamental chemical principles. The recognition of carbon's oxidation state as +2 in CO3 represents a triumph of scientific inquiry, underscoring the dynamic nature of chemical knowledge and the continual evolution of our comprehension of molecular behavior.
This paradigm-shifting discovery transcends the confines of theoretical inquiry, permeating diverse fields and catalyzing a renaissance of scientific inquiry. The redefined oxidation state of carbon in CO3 not only challenges established dogma but also propels us toward new frontiers of discovery, inspiring fresh perspectives on the electronic intricacies of chemical species and the dynamic interplay of electrons in molecular systems.
The implications of this transformative revelation extend far beyond the realm of theoretical chemistry, permeating environmental science, industrial processes, and educational curricula. The redefined understanding of carbon's oxidation state in CO3 holds implications for the understanding of carbon cycling in the environment, impacting fields such as carbon sequestration, ocean chemistry, and the dynamics of carbonate minerals in geological systems.
Furthermore, the recognition of carbon's oxidation state as +2 in CO3 unlocks a spectrum of technological and industrial applications, inspiring novel approaches in materials science, catalysis, and chemical synthesis. This transformative insight has the potential to catalyze innovations in diverse industrial sectors, from the production of specialty chemicals to the advancement of environmentally benign processes that leverage the reactivity of carbonate-based compounds.
In essence, the unveiling of carbon's oxidation state as +2 in the carbonate ion (CO3) marks a pivotal juncture in the annals of chemical discovery, redefining our understanding of a fundamental polyatomic species and catalyzing a paradigm shift in our comprehension of the electronic intricacies that underpin chemical reactivity and molecular behavior. As we embark on the next phase of scientific exploration, the revelations that await promise to reshape our understanding of chemical bonding and catalyze new avenues of scientific inquiry.